Innate immunity, epigenetics and metabolism
Sepsis occurs when body's immune response to infection becomes harmful. With more than 25 million cases and 8 million deaths per year worldwide, sepsis is one of the most common yet least recognized illness. In May 2017, the World Health Assembly and the World Health Organization (WHO) made sepsis a global health priority by adopting a resolution to improve the prevention, diagnosis and management sepsis. In this context, the aim of our work is to deepen our knowledge of the mechanisms regulating anti-infectious immune responses, in order to develop new tools for the diagnosis and treatment of sepsis.
Main Research Topic
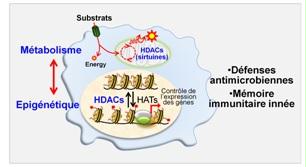
Metabolism, epigenetic and anti-microbial host defenses: the role of histone deacetylases
To prevent and fight infections, our body has an efficient defence system, the innate immune system. Upon pathogen sensing, cells of the innate immune system adapt their metabolism to meet the energy requirements necessary for defence functions. This metabolic adaptation is associated with epigenetic changes that affect gene structure and entourage without changing their sequence. A major recent discovery in immunology, breaking the dogma of immune memory by adaptive cells only, has been to show that metabolic and epigenetic changes are associated with the acquisition of a form of memory by innate immune cells. Moreover, aberrant epigenetic and metabolic changes are observed in sepsis patients.
Histone deacetylases (HDACs) are involved in the control of metabolic and epigenetic processes. We study the impact of drugs or natural products (produced in our intestines) targeting HDACs as well as that of deficiency in certain HDACs (especially sirtuins) on inflammatory and anti-infectious responses as well as on sepsis development. We are also testing the extent to which HDACs participate in the establishment of memory by innate immunity cells (also known as “innate immune training”).
- Roger et al., Blood. 2011;117(4):1205
- Mombelli et al., J Infect Dis. 2011;204(9):1367
- Ciarlo et al., Int J Antimicrob Agents. 2013;42 Suppl:S8
- Ciarlo et al., Sci Rep. 2016;6:37944
- Ciarlo et al., Sci Rep. 2017;7(1):3853.
- Ciarlo et al.,Front Immunol. 2017;8:1037
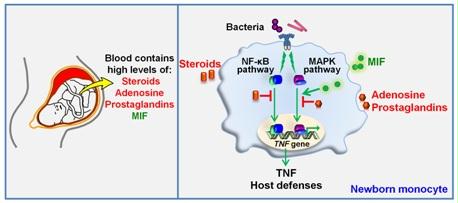
Neonatal innate immunity
Nearly 3 million newborns die each year. Infections are the second leading cause of neonatal mortality (i.e. during the first 4 weeks of life). The susceptibility of newborns to infections is associated with their limited ability to develop effective innate immune responses. In collaboration with Dr Giannoni from the Service of Neonatology of the CHUV, we analyse the mechanisms underlying the high susceptibility to infections of newborns.
Fetal and neonatal blood contains high concentrations of anti-inflammatory mediators such as steroid hormones, adenosine and prostaglandins (left). We reported that placental derived steroids are strong immunosuppressors of innate immune responses of newborn monocytes through inhibition of the NF-κB pathway (right). We also observed that the proinflammatory cytokine MIF (macrophage migration inhibitory factor) circulates in the bloodstream of neonates at concentrations 10-fold higher than in infants and adults. These elevated MIF levels counter-regulate the immunosuppressive activity of adenosine and prostaglandins at the level of MAPKs. Our data challenge the concept that the neonatal immune system is purely immature and suggest the existence of mechanisms counterbalancing neonatal immunosuppression.
- Giannoni et al., Infect Immun. 2011;79(7):2690
- Schlapbach et al., Intensive Care Med. 2013;39(4):754
- Roger et al., Proc Natl Acad Sci U S A. 2016;113(8):E997
- Giannoni et al., Méd Sci (Paris). 2016;32(12):1062
- Roger et al. Front Immunol. 2017;8:26
Identification of biomarkers and therapeutic targets for sepsis
Sepsis is a clinical syndrome defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection (Sepsis-3 definition released in JAMA in 2016). Sepsis heterogeneity is a major reason why clinical trials testing immunomodulatory drugs failed to confer benefit to patients. Whether inflammatory cytokines, immunoparalysis or metabolism should be targeted should be anticipated at the individual level, suggesting that, sepsis is a field in which personalized medicine could be applied for patient’s benefit. Through international collaborations (Horizon 2020 Marie-Skłodowska-Curie Innovative Training Network), we aim to develop and validate genetic, molecular and cellular biomarkers that can enable development and implementation of personalized treatment strategies of sepsis
- Roger et al., Proc Natl Acad Sci U S A. 2009;106(7):2348
- Kerschbaumer et al., J Biol Chem. 2012;287(10):7446
- Renner et al., FASEB J. 2012;26(2):907
- Savva & Roger, Front Immunol. 2013;4:387
- Herderschee et al., J Leukoc Biol. 2015;98(1):23
- Stalder et al., PLoS One. 2016;11(10):e0163542
- Savva et al., Proc Natl Acad Sci U S A. 2016;113(13):3597
- Also see : http://europeansepsisacademy.com/
Contacts
Lausanne University Hospital (CHUV)
CLED.04.407
Chemin des Boveresses 155
CH-1066 Epalinges
Switzerland



