Safety of pacemakers and other devices
In 1997, the development of MR-safe devices was initiated by J. Schwitter by teaming up with the ETH Zurich, Medtronic, and the Electrophysiology Unit of the Cardiology Division (University Hospital Zurich).
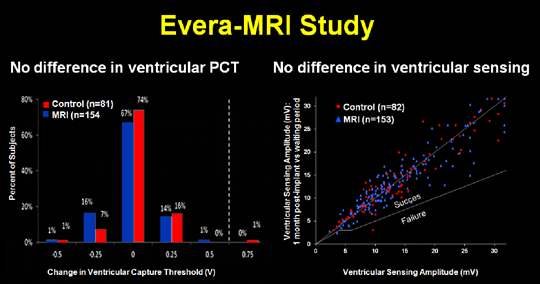
This research was the first to bring an MR-conditional pacemaker to the market. Several multicenter trials such as the “Advisa-MRI” trial (35 centers; Gimbel, Heart Rhythm 2013; Schwitter, Heart Rhythm 2013), the “5076-MRI” trial (36 centers), and the “Evera-MRI” trial (42 centers world-wide; Gold JACC 2015) proved the safety of MR-conditional devices (J. Schwitter contributing as a steering committee member, scan advisory and publication committee member to these studies).
In 1997, the development of MR-safe devices was initiated by J. Schwitter by teaming up with the ETH Zurich, Medtronic, and the Electrophysiology Unit of the Cardiology Division (University Hospital Zurich).
This research was the first to bring an MR-conditional pacemaker to the market. Several multicenter trials such as the “Advisa-MRI” trial (35 centers; Gimbel, Heart Rhythm 2013; Schwitter, Heart Rhythm 2013), the “5076-MRI” trial (36 centers), and the “Evera-MRI” trial (42 centers world-wide; Gold JACC 2015) proved the safety of MR-conditional devices (J. Schwitter contributing as a steering committee member, scan advisory and publication committee member to these studies).
Publications
A total of 73 articles were published as a result of the CRMC research activity since its foundation in 2009.
All publicationsContacts
Director CMR Center
Rue du Bugnon 46
1011 Lausanne



