HPV and Cervical Cancer
HPV are non enveloped, small doubled-stranded DNA viruses that are strictly epitheliotropic. About 40 HPV types infect the anogenital tract. Low risk HPV types induce benign disease, such as genital warts (90 % caused by HPV 6 and 11), while the high risk types are associated with malignant progression (pre-neoplastic lesions such as cervical intraepithelial neoplasia, CIN that can progress to cancer). The majority of adenocarcinomas and carcinomas of the cervix and of squamous cell cancers (SCC) of the vulva, vagina, penis and anus are caused by HPV-16 and HPV-18 which together account for about 70% of cases. The remaining 30% of ano-genital cancers are associated with other high-risk HPV types such as HPV-31, -33, -35, -39, -45,-51, -52, -58...
Functions of viral genes
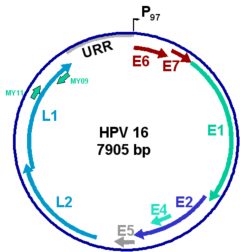
The HPV genome encodes early proteins with regulatory (E1, E2) and transforming functions (E6 and E7) and the structural capsid proteins (L1 and L2). E1 and E2 are expressed in the epithelial basal cells and facilitate HPV episome replication and maintenance, while the E6 and E7 transforming proteins are expressed in replicating suprabasal cells. Exponential viral DNA synthesis and production of the capsid proteins take place in the upper layers of the infected epithelium. Virion assembly occurs only in the squamous layers, and mature progeny virus is released with the keratin squames.
The L1 gene carries a conserved region, shown here flanked by the MY09 and MY11 primer sites, that is targeted in PCR assays aimed at identifying and typing HPV in clinical samples. For established cancer cases, assays targeting the E6/E7 region may be preferred as this region is always present in malignant tumors (see below).
The viral productive cycle of HPV is linked to the differentiation program of keratinocytes. In condylomas, papillomas and mild to moderate dysplasia, viral DNA is episomal, and production of virus can occur. In high-grade dysplasia and cancer lesions, the viral DNA gets integrated into the host cell chromosomes. Integration is random in the chromosomes but frequently occurs in the early region of the viral DNA, disrupting the E2 open reading frame with concomitant deregulated expression of the viral oncoproteins E6 and E7. The E7 protein is thought to induce cell proliferation and disrupt the cell cycle regulation by inactivation of proteins of the retinoblastoma family, whereas E6 blocks cell apoptosis by targeting the p53 tumor suppressor protein to degradation via the proteasome pathway. E7 and E6 are the only HPV proteins consistently expressed in cervical cancers, and therefore represent the major tumor antigens targeted by immunotherapy. HPV-positive cancer cells are non permissive for virus production, express markers of cellular proliferation, and present chromosomal rearrangements.
The development of an anogenital cancer lesion is a rare and slow multi-step process associated with a persistant infection with the same high risk HPV: from mild to moderate dysplastic lesions to high-grade squamous intraepithelial lesions (HSIL) and cancer that takes more than 10-15 years.
Vaccines
Prophylactic HPV vaccines aim at eliciting neutralizing antiviral antibodies to prevent HPV infection in naïve individuals, while therapeutic vaccine candidates aim at inducing cell-mediated immune responses to eliminate HPV-infected and/or transformed tumor cells in already infected patients.
Given widespread infection of HPV and lack of antiviral agents against HPV, the development of prophylactic vaccines has been a long-sought strategy to prevent cervical cancer. It has been demonstrated that the major papillomavirus capsid protein L1 has the intrinsic ability to self-assemble into Virus-Like Particles (VLPs) which resemble HPV virion but are devoid of genetic material. These VLPs can be produced in baculovirus-infected insect cells, in yeast, or in other cell substrates. They induce high titers of virus-neutralizing antibodies even in the absence of an adjuvant. In preclinical studies, vaccination of animals resulted in excellent protection from homologous virus challenge, and passive transfer of antibodies from the vaccinated animals also conferred protection, confirming the importance of neutralizing antibodies.
Two leading prophylactic vaccines have been developed, one by GlaxoSmithKline (Cervarix®) that contains HPV 16 and 18 VLPs and one by Merck ( Gardasil®) that contains HPV 16, 18, 6 and 11 VLPs. These vaccines are administered by intramuscular injections (3 doses at 0, 1-2 and 6 months) and induce type-specific HPV-neutralizing antibodies. Recent efficacy trials have shown that these prophylactic vaccines can prevent persistent type-specific HPV infection and associated lesions.
A major challenge will now be to achieve the worldwide implementation of these vaccines, particularly in developing countries, which account for more than three-quarters of the worldwide cases of cervical cancer.



